Prescribing Information
This site is intended for US Healthcare Professionals only
Journal of Urology (LOCATE study)
A prospective, US, multicenter, open-label study investigating the impact of Axumin® (fluciclovine F 18) PET/CT imaging on patient management of biologically recurrent prostate cancer after initial prostate cancer treatment and after negative or equivocal findings on standard-of-care imaging.1

Primary endpoint: The percentage of men with biochemical recurrence of prostate cancer following initial prior therapy whose clinical management was changed following an Axumin PET/CT scan as recorded by completed form stating their intended management before and after the scan, with any changes recorded.1
When presented with a negative or an equivocal scan, Axumin imaging may provide clarity for your decision-making1

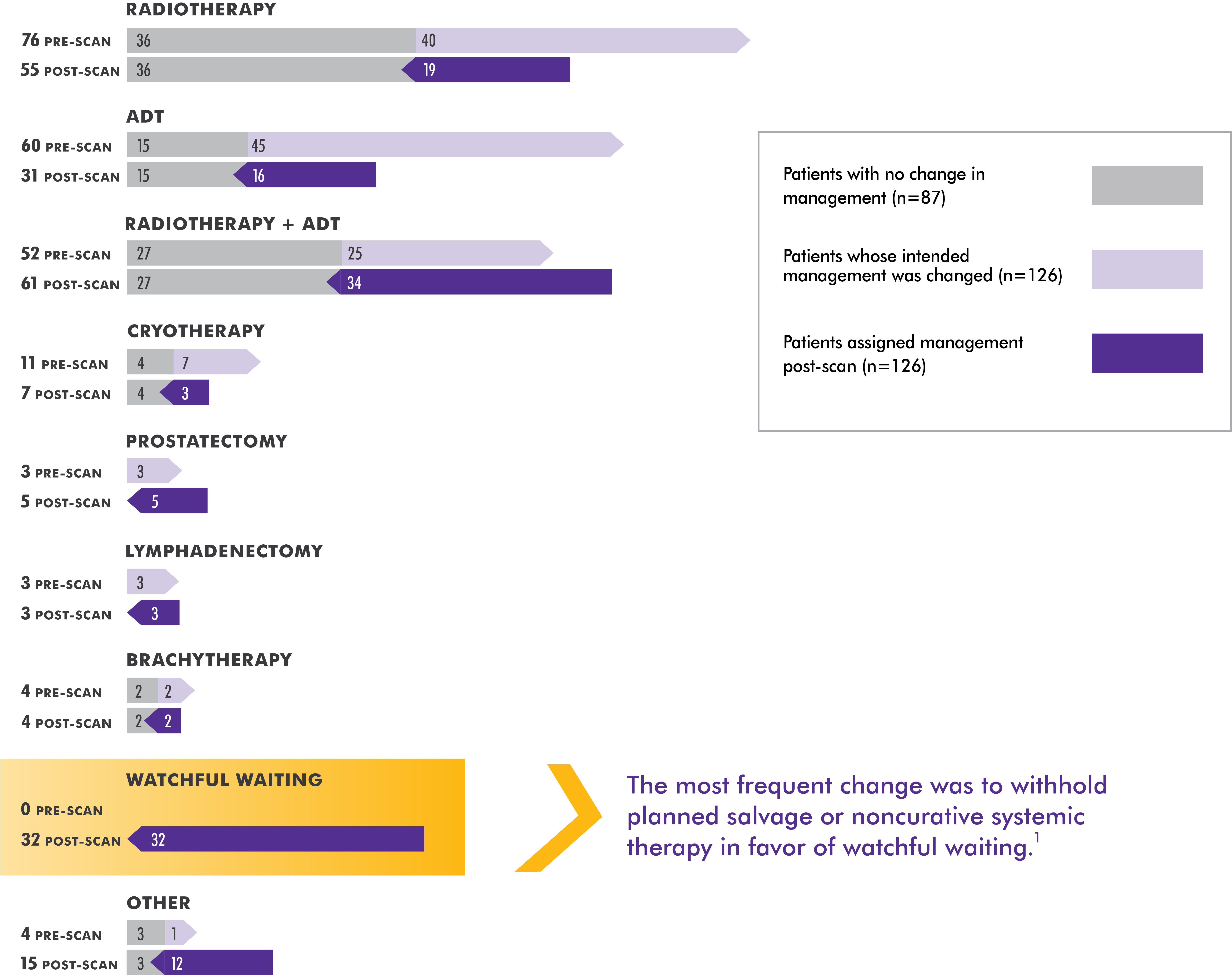
The specific treatment plan selected after the Axumin PET/CT imaging results were available was based on the independent judgment of the study investigators, who utilized any other available confirmatory information. The clinical utility of Axumin PET/CT to identify a particular course of treatment has not been established and clinical correlation, including potential histopathological evaluation of the suspected recurrence site, is recommended.
Axumin-avid lesions were detected in 57% of patients (122/213) who had a negative equivocal finding on standard-of-care imaging, with 30% seen in the prostate or prostate bed and 38% outside (including 29% in lymph nodes, 2.3% soft tissues, and 11% bone).1
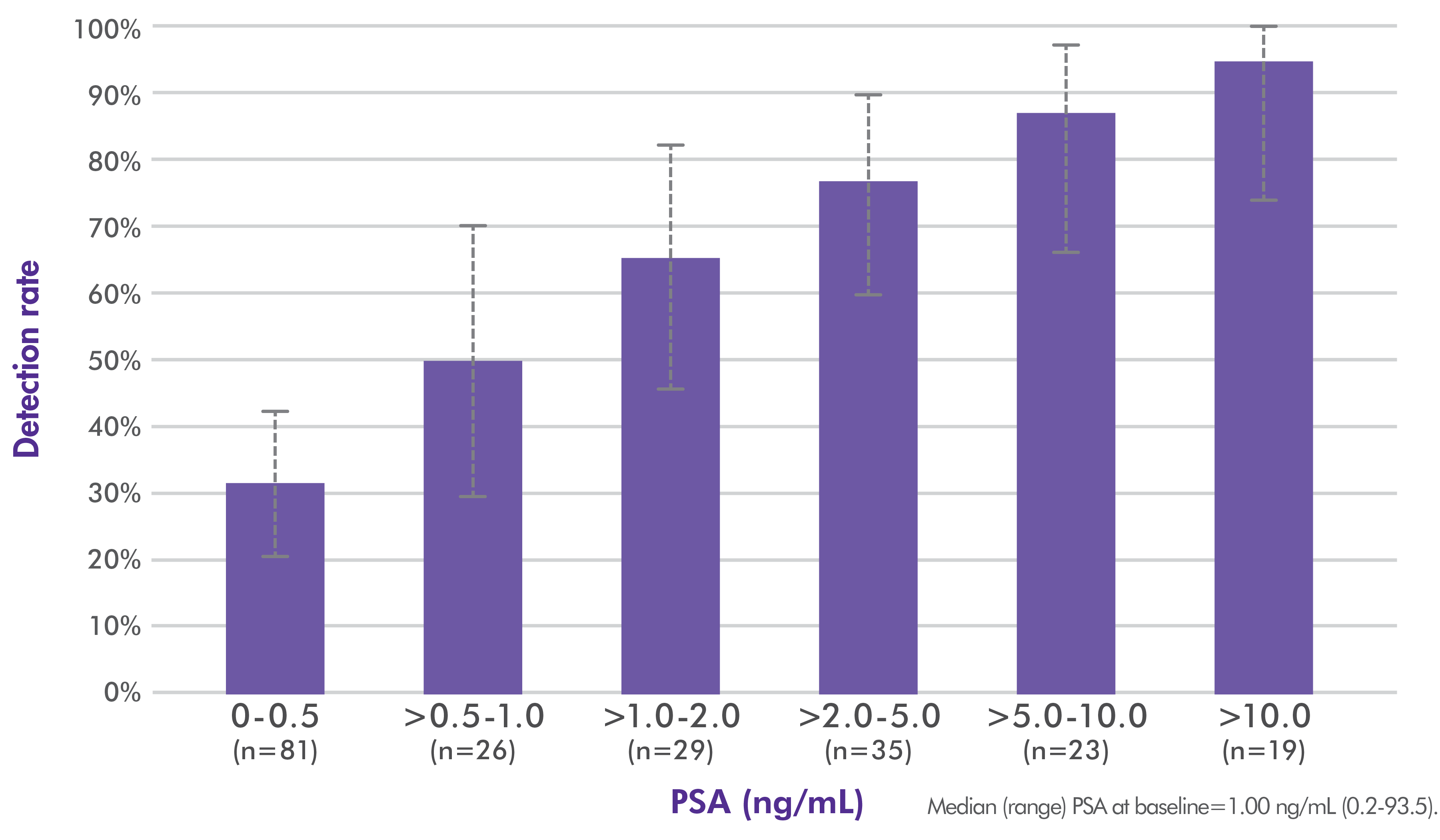
Axumin whole-body imaging detection rate by PSA level1
The safety profile of Axumin in the LOCATE trial was consistent with that described in the approved US Prescribing Information.
Lancet (EMPIRE-1 study)
A single-center, open-label, phase 2/3, randomized study comparing 3-year event-free survival in patients receiving radiotherapy based on conventional imaging vs Axumin PET/CT imaging. Eligible patients had adenocarcinoma of the prostate following prostatectomy, detectable PSA, and no systemic metastases on conventional imaging.3*

*Conventional imaging included a bone scan and either a CT or an MRI of the abdomen and pelvis.
Primary endpoint: 3-year event-free survival.
Event was defined as:
Secondary endpoints: Impact of Axumin in post-prostatectomy radiotherapy decision-making, volumetric and dosimetric changes, and gastrointestinal and genitourinary toxicity.
Prespecified in treatment decision based on PET findings:
†The boost consisted of 45 to 50.4 Gy to pelvic nodes and 64.8 to 70.2 Gy to the prostate bed.
ADT, androgen deprivation therapy; CT, computed tomography; MRI, magnetic resonance imaging; XRT, radiation therapy.
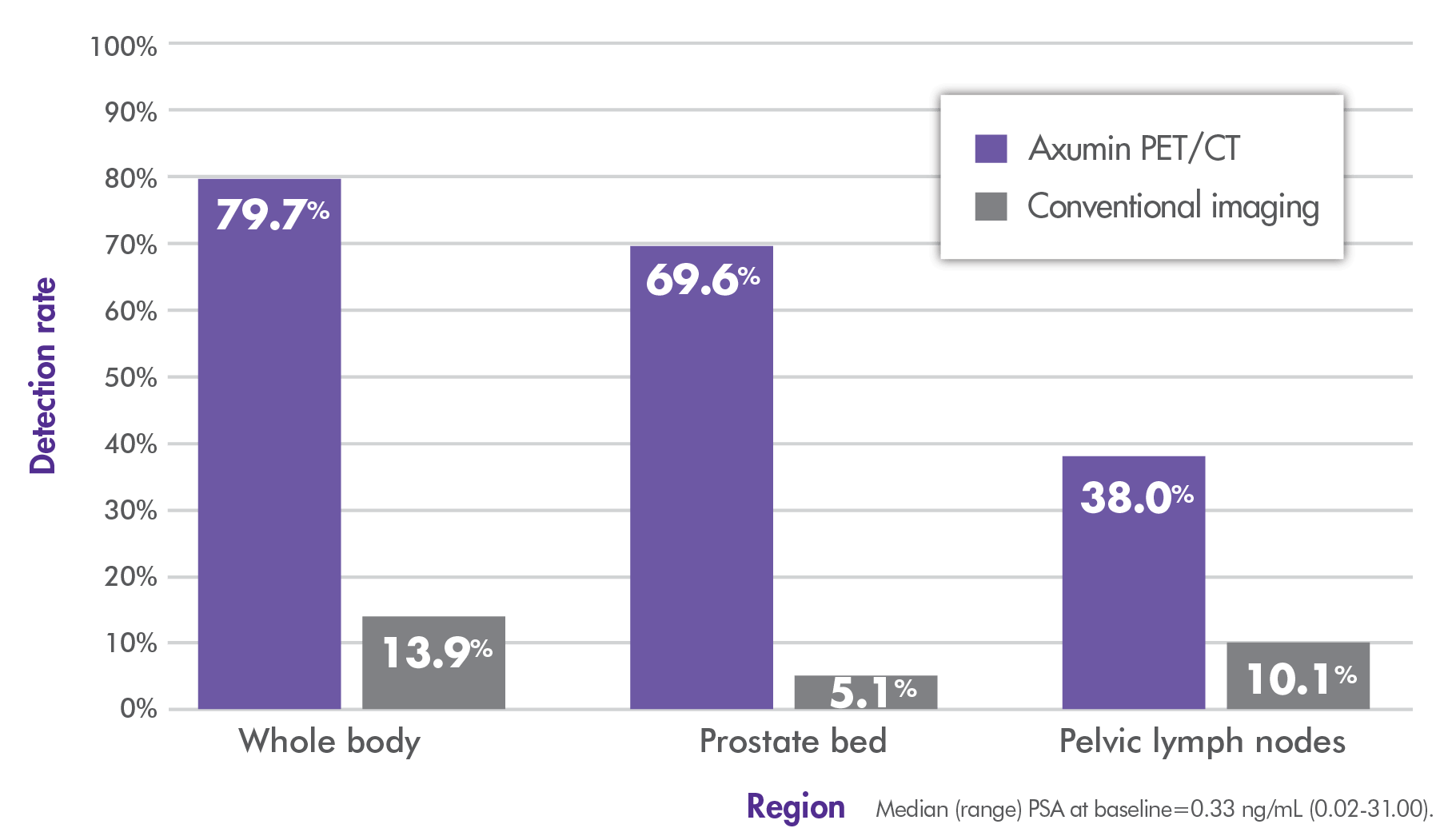
~80% overall (whole-body) detection rate with Axumin imaging vs 14% with conventional imaging.3,4
Axumin PET/CT changed clinical decision-making in more than one-third of patients

There were no significant differences in toxicity across both study arms3
Abbreviation: ADT, androgen deprivation therapy.
Axumin® (fluciclovine F 18) injection is indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment.
To report suspected adverse reactions to Axumin, call 1-855-AXUMIN1 (1-855-298-6461) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Axumin full Prescribing Information.
You are now leaving Axumin.com. Do you wish to proceed?