Prescribing Information
This site is intended for US Healthcare Professionals only
Start scanning in just 3-5 minutes. Total scan time is typically 20-30 minutes.

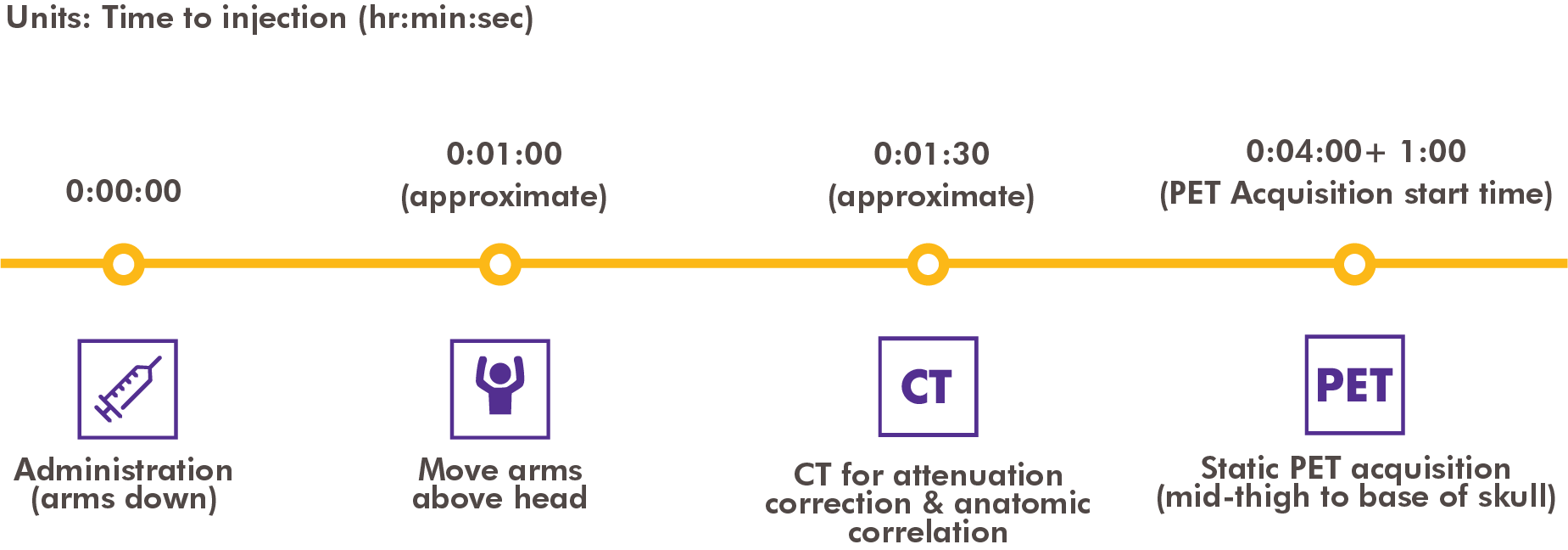
Axumin is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure during administration. The recommended dose is 370 MBq (10 mCi) administered as an intravenous bolus injection.1
Patient Preparation Prior to PET/CT Imaging: Advise the patient to avoid any significant exercise for at least one day prior to PET/CT imaging, do not eat or drink for at least four hours (other than sips of water for taking medications) prior to administration of Axumin, and void approximately 30 minutes to 60 minutes before injection of Axumin and then refrain from voiding until after the scan has been completed.

Download the Axumin patient brochure.
Position the patient supine with arms above the head.1 If the patient cannot tolerate this position for the duration of the study, an alternate position for the patient’s arms may be used.
Begin PET scanning 3 to 5 minutes after completion of the Axumin injection.1 Following intravenous administration, the tumor-to-normal tissue contrast is highest between 4 and 10 minutes after injection with a 61% reduction in mean tumor uptake at 90 minutes after injection.1
High quality CT acquisition for anatomic correlation and attenuation correction is recommended. It is recommended that image acquisition should start from mid-thigh and proceed to the base of the skull.1 Typical total scan time is between 20 to 30 minutes.1 Identification of tumor location is based on fluciclovine F 18 uptake in comparison with tissue background. For larger lesions (≥1 cm), uptake equal to or greater than bone marrow is considered suspicious for prostate cancer recurrence. For small lesions (<1 cm), focal uptake greater than blood pool should be considered suspicious for prostate cancer recurrence.1
Axumin® (fluciclovine F 18) injection is indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment.
To report suspected adverse reactions to Axumin, call 1-855-AXUMIN1 (1-855-298-6461) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Axumin full Prescribing Information.
You are now leaving Axumin.com. Do you wish to proceed?