Prescribing Information
This site is intended for US Healthcare Professionals only
Fluciclovine F 18:
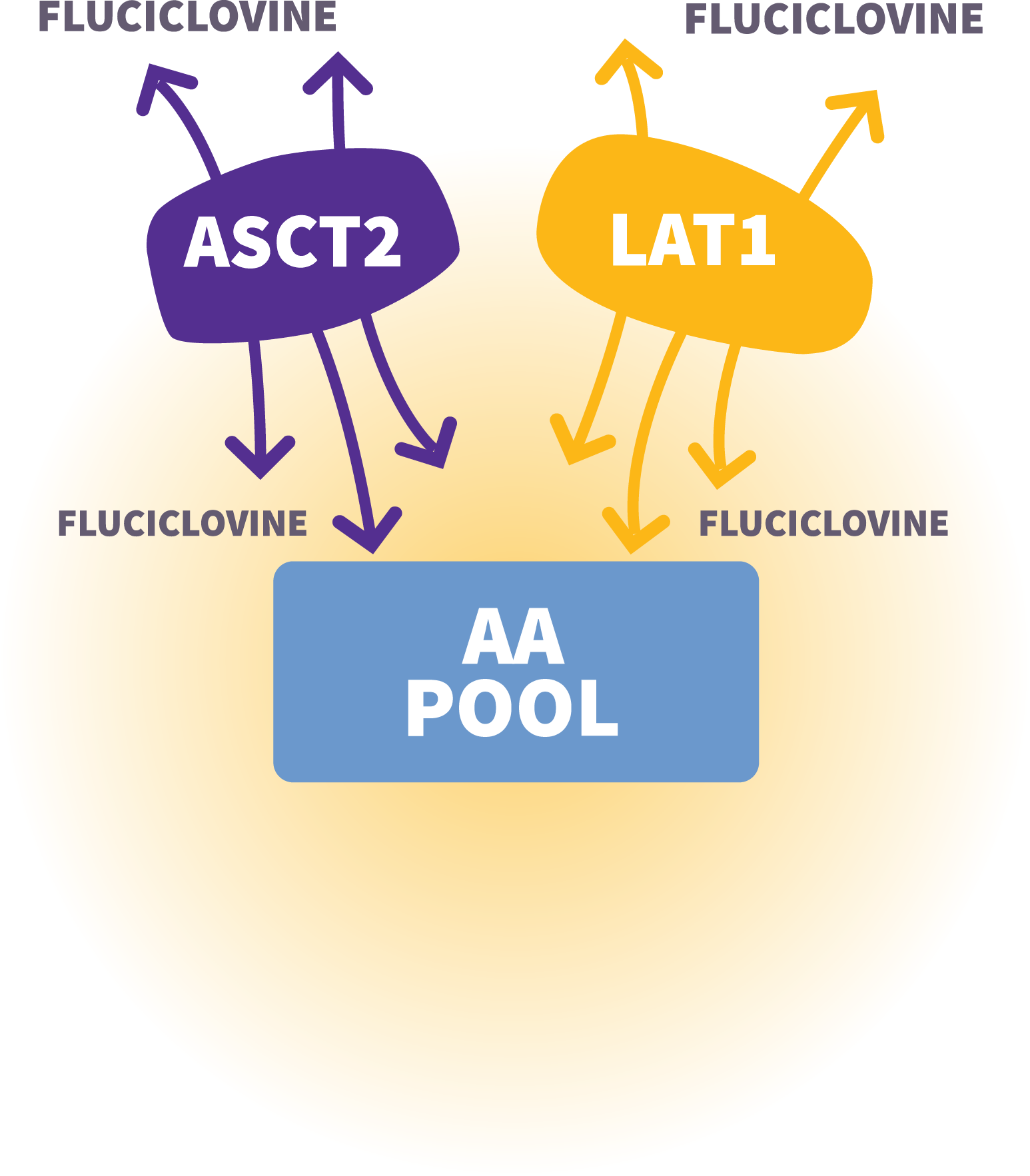
Axumin mechanism of action: Increased uptake of fluciclovine into prostate cancer cells by upregulated amino acid transporters, such as ASCT2 and LAT1.1-4
Adapted from Fuchs BC. Semin Cancer Biol. 2005.4
Axumin® (fluciclovine F 18) injection is indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment.
To report suspected adverse reactions to Axumin, call 1-855-AXUMIN1 (1-855-298-6461) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Axumin full Prescribing Information.
You are now leaving Axumin.com. Do you wish to proceed?