Prescribing Information
This site is intended for US Healthcare Professionals only
The purpose of this web page is to offer information regarding reimbursement for Axumin. Reimbursement information and processes will evolve over time, as they do with any prescription drug. Please revisit this website periodically for the most up-to-date information.
Telephone: 1-855-495-9200
FAX: 1-877-309-7514
Telephone support is available Monday through Friday, from 9 AM to 8 PM Eastern time.
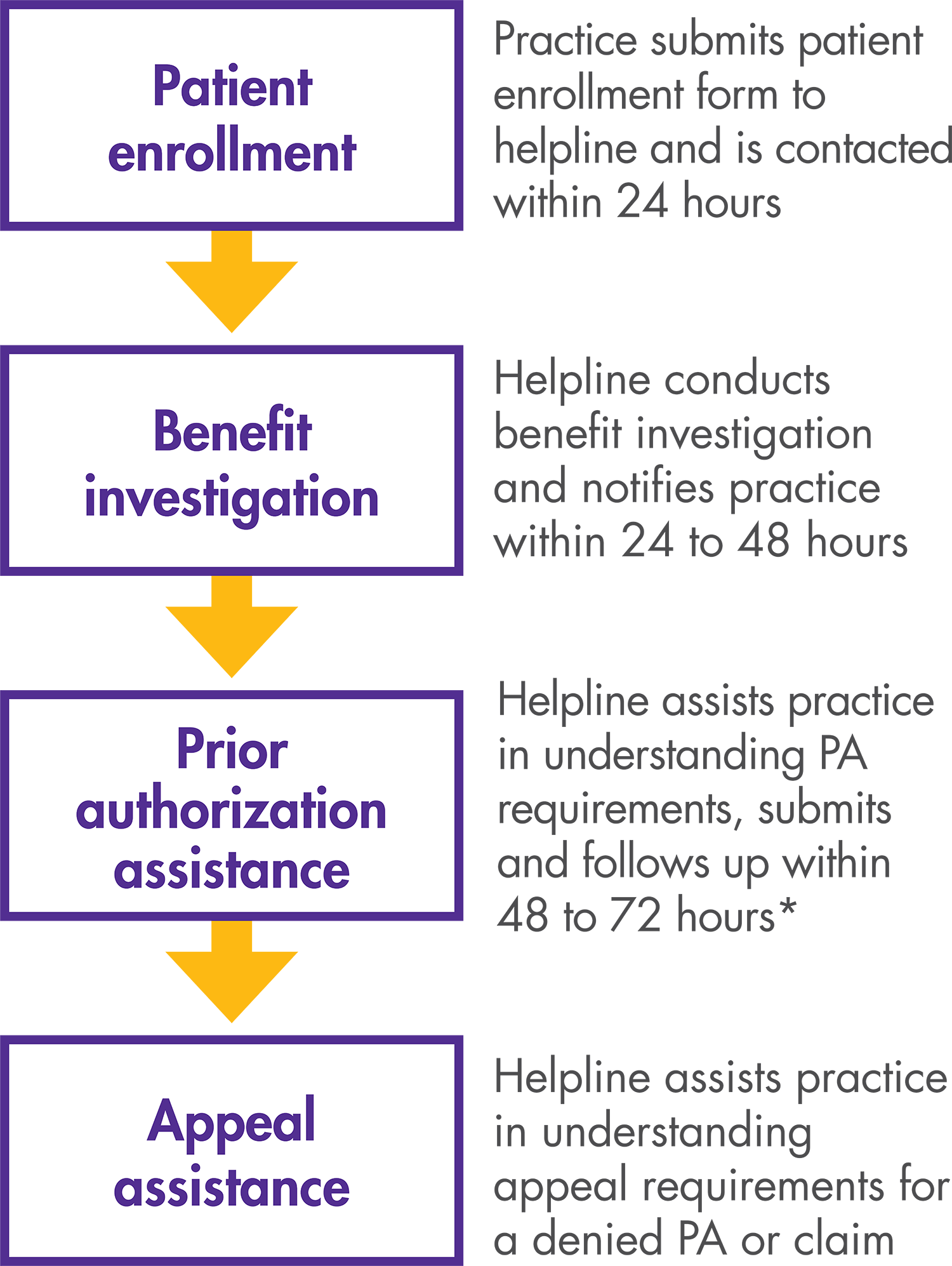
*Helpline provides information about PA/appeals requirements, and, at the provider’s option, submits PA forms completed by the provider to the payer.
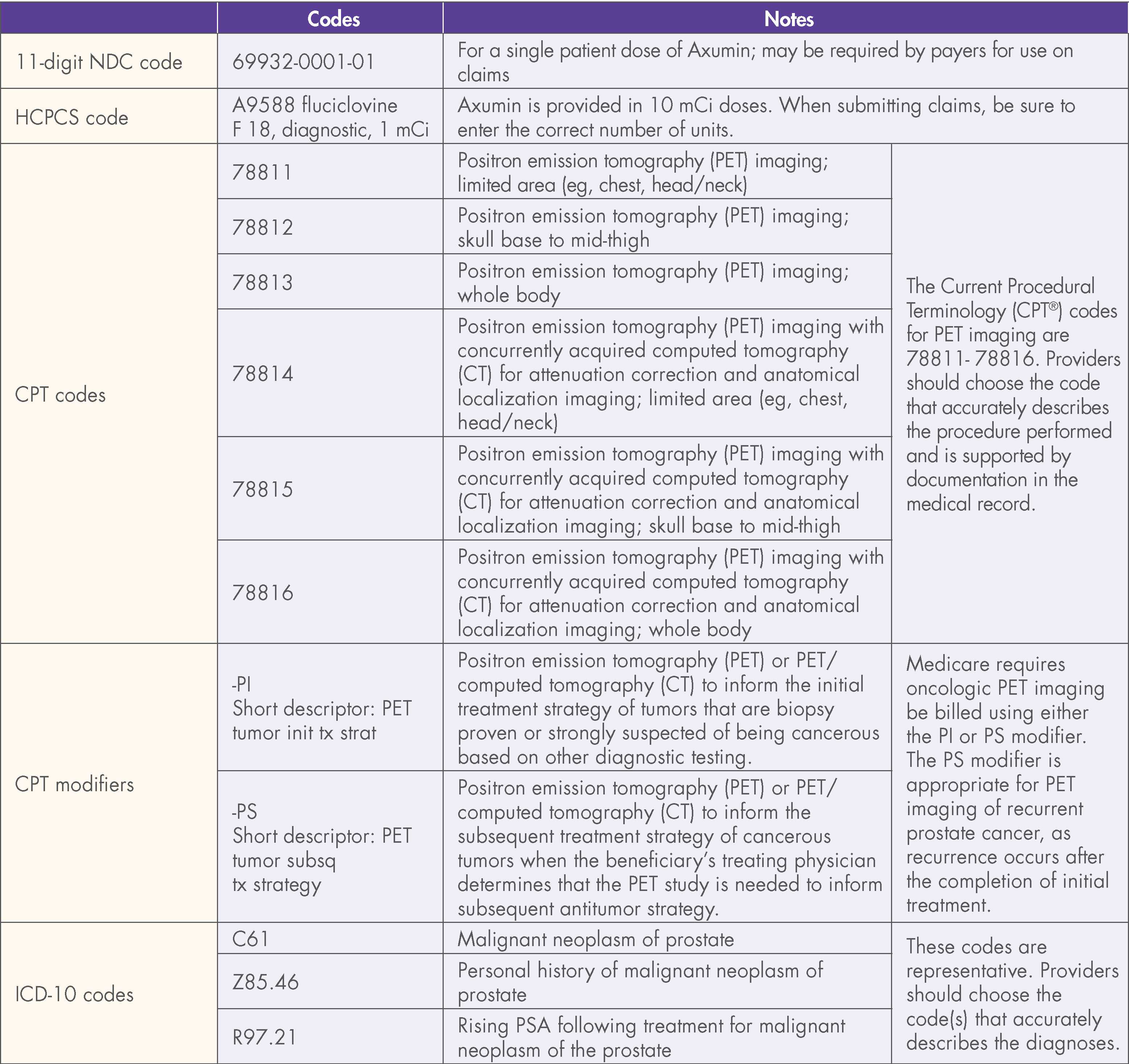
This document contains factual information and is not intended to be legal or coding advice. Blue Earth Diagnostics does not guarantee coverage or reimbursement for Axumin. The information provided in this document is based upon current, general coding practices. The existence of billing codes does not guarantee coverage and payment. Payer policies vary and may change without notice. It is the providers’ responsibility to determine and submit accurate information on claims. This includes submitting such as proper codes, modifiers, charges, and invoices for the services that were rendered. The coding on claims should reflect medical necessity and be consistent with the documentation in the patient’s medical record.
Axumin's transitional pass-through period for Original Medicare beneficiary scans performed in the hospital outpatient department ended on December 31, 2019. For Medicare hospital outpatients, payment for the PET diagnostic radiopharmaceuticals are bundled with the procedure payment after the transitional pass-through period. As of January 1, 2020, Axumin will be bundled with the procedure payment.
It is essential that hospitals appropriately and accurately determine codes for items and services and apply appropriate charges, even when the payment is bundled. For example, diagnostic radiopharmaceuticals are packaged but still should be coded and billed in order for the cost to be accurately represented in the claims data.
Download all formulary components or choose one or more of the individual components below.
The information on this website contains factual information and is not intended to be legal or coding advice. Blue Earth does not guarantee coverage or reimbursement for Axumin. The existence of billing codes does not guarantee coverage and payment. Payer policies vary and may change without notice. It is the provider’s responsibility to determine and submit accurate information on claims. This includes submitting proper codes, modifiers, charges, and invoices for the services that were rendered. It is the provider’s responsibility to ensure that all information on a claim is accurate. It is the provider’s responsibility to check with the payer to determine whether the information contained on the claim is accurate. It is the responsibility of the provider to document the medical necessity of Axumin in the medical record.
Axumin® (fluciclovine F 18) injection is indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment.
To report suspected adverse reactions to Axumin, call 1-855-AXUMIN1 (1-855-298-6461) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Axumin full Prescribing Information.
You are now leaving Axumin.com. Do you wish to proceed?